-
PDF
- Split View
-
Views
-
Cite
Cite
Irja Davidkin, Sari Jokinen, Mia Broman, Pauli Leinikki, Heikki Peltola, Persistence of Measles, Mumps, and Rubella Antibodies in an MMR-Vaccinated Cohort: A 20-Year Follow-up, The Journal of Infectious Diseases, Volume 197, Issue 7, 1 April 2008, Pages 950–956, https://doi.org/10.1086/528993
Close - Share Icon Share
Abstract
BackgroundThe persistence of antibodies against measles, mumps, and rubella induced by the measles-mumps-rubella (MMR) vaccine and the kinetics of antibody decline after the second MMR vaccine dose were studied in the same cohort for 20 years
MethodsMeasles, mumps, and rubella antibodies were measured by enzyme immunoassay in 20-year follow-up serum samples (n=183) of twice-vaccinated individuals, and measles antibodies were also measured in oral fluids (n=177). Antibody decay was determined in a group (n=58) with subsequent samples collected 1, 8, and 15 years after the second MMR dose
ResultsIn total, 95%, 74%, and 100% of 183 vaccinees were still seropositive for measles, mumps, and rubella, respectively, and 85% of 177 vaccinees had measurable measles antibodies in their oral fluids. The antibody levels declined significantly after the second dose, but subsequently the rate of decline was slower
ConclusionsA high rate of seropositivity was found 20 years after the first MMR dose, particularly for rubella and measles. Our results show that MMR vaccine–induced antibodies wane significantly after the second dose. According to epidemiological data, the protection induced by MMR vaccination in Finland seems to persist at least until early adulthood. However, the situation requires constant vigilance
The vaccines against measles, mumps, and rubella have been in use for >30 years as both single and trivalent vaccines. An increasing number of countries have significantly reduced the incidence of measles, mumps, and rubella and have even reached an elimination phase through successful use of the measles-mumps-rubella (MMR) vaccine [1–3]. Protective levels of antibodies induced by the MMR vaccine were first suggested to be lifelong; however, the levels of measles, mumps, or rubella antibodies have been shown to decline over time, faster after vaccinations than when naturally acquired [4, 5]
Experience about the persistence of MMR vaccine–induced immunity has accumulated from countries with various epidemiological situations and vaccination policies [6–13]. In some of these countries, wild measles, mumps, and rubella viruses still circulate, even to a small extent, and may influence the persistence of MMR vaccine–induced antibodies [14]
We have followed the kinetics of antibody persistence in the same MMR-vaccinated cohort in repeated samples obtained since 1982 and found that all of the antibodies induced by MMR vaccine wane over time [15–17]. This was shown to happen especially when the incidence of measles, mumps, and rubella became very low in Finland and natural diseases did not boost the once-gained antibody levels
In Finland, the incidence of measles, mumps, and rubella declined soon after the MMR vaccination campaign was launched [3]. In 1982, 1986, and 1990, the incidence of measles was 112, 15, and 0.9 cases/100,000 population, respectively; the incidence of mumps was 43, 11, and 0.4/100,000; and the incidence of rubella was 64, 21, and 6/100,000. Since the beginning of the 1990s, measles, mumps, and rubella have been eliminated from Finland, and only a few imported cases have occurred annually [1, 2]. This disease-free period of >10 years allows us to study the persistence of vaccine-induced antibody levels alone
We report here the present antibody status of the MMR-vaccinated cohort. The measles, mumps, and rubella antibody levels were measured in serum and oral fluids obtained 20 years after the first MMR dose. In addition, we determined the rate of antibody decline, also using the samples collected previously from our cohort. Furthermore, measles, mumps, and rubella antibody levels were compared according to sex and place of residence of the vaccinees
Subjects, Materials, and Methods
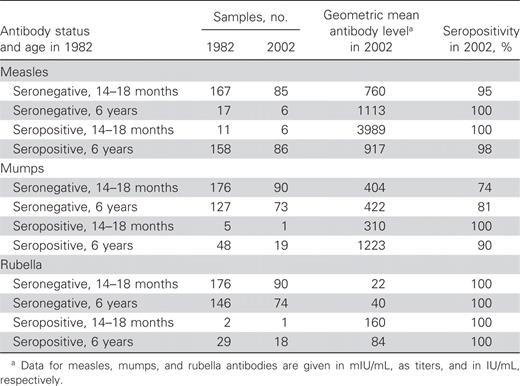
Cohort and vaccinationsThe MMR-vaccinated cohort of 353 children (158 girls and 195 boys) initially comprised 2 age groups: 14–18 months old (the younger group; n=178) and 6 years old (the older group; n=175). The cohort was recruited from 3 cities, located in eastern (Joensuu), western (Pietarsaari), and northern (Ivalo) Finland. A large majority of children were seronegative when vaccinated at the age of 14–18 months. Of the 178 younger vaccinees, 11 (6.2%), 5 (2.8%), and 2 (1.1%) already had antibodies against measles, mumps, and rubella, respectively, before MMR vaccination. Of the 175 older vaccinees, 158 (90%) were already seropositive for measles as a result of natural disease or monocomponent measles vaccination, and 48 (27%) and 29 (17%) were seropositive for mumps and rubella, respectively. The numbers of samples in the different subgroups are shown in table 1
Seropositivity, samples sizes, and antibody levels in 2002, by subgroup
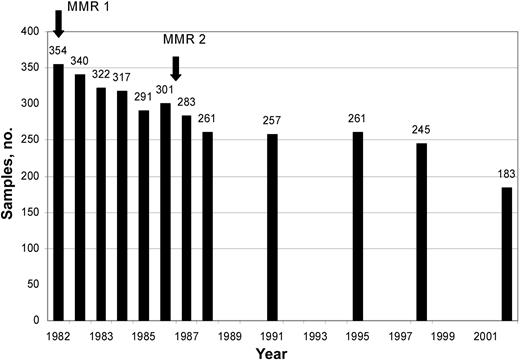
The first MMR vaccination was given in 1982 to all members of the cohort. The second dose was given to the younger group at the age of 6 years (1987) and to the older group at the age of 11–13 years (1987–1989). The vaccine used for both vaccinations was M-M-R II vaccine (containing the Moraten strain of measles virus, the Jeryl Lynn strain of mumps virus, and the RA27/3 strain of rubella virus; Merck). The number of serum samples collected during the entire follow-up is shown in figure 1
No. of samples collected from the cohort in different years during follow-up, from 1982 to 2002. The arrows indicate the times of the measles-mumps-rubella (MMR) vaccinations
Follow-up samples in 2002In 2002, 20 years after the first and 13–15 years after the second MMR vaccine dose, all members of the cohort were invited by letter to participate in a study determining their measles, mumps, and rubella antibody levels. Serum samples were collected from 183 vaccinees (for the younger group, n=91; for the older group, n=92). In addition, we collected oral fluids from 178 vaccinees, using an OraSure device (OraSure Technologies). In 2002, the vaccinees were 22 (younger group) or 26 (older group) years old. The blood samples and oral fluids were collected by special appointment, in hospital laboratories or in health care centers. The study was approved by the Hospital District of Helsinki and Uusimaa Ethics Committee of Epidemiology and Public Health. All vaccinees gave written consent before their samples were collected
Determination of antibody declineThe numbers of samples received at different time points varied during follow-up (figure 1), because samples were collected on a voluntary basis. For this reason, to make a proper determination of the rate of antibody decline after the second vaccine dose, we tested samples from 58 (of 178) younger vaccinees who had been seronegative at the first vaccination and from whom samples were available from the years 1987, 1995, and 2002, collected 5, 12, and 20 years after the first dose and 1, 8, and 15 years after the second dose, respectively. The samples were retested in parallel using the same lots of immunoassays, and antibody levels were used to determine the decay rates
Antibody testsMeasles, mumps, and rubella IgG antibodies were measured by use of a commercial EIA (Enzygnost; Dade Behring), in accordance with the manufacturer’s instructions. We evaluated qualitative results according to the following cutoff values: optical density (OD) <0.100, negative; OD from 0.100 to 0.200, equivocal; and OD >0.200, positive. Antibody levels were calculated using the α method [18], in accordance with the manufacturer’s instructions. The quantitative cutoff value for seropositivity was ⩾150 mIU/mL for measles, a titer of ⩾1:230 for mumps, and ⩾4 IU/mL for rubella. The antibody levels were defined as low positive (equivocal) at 150–350 mIU/mL for measles, a titer of 1:230 to 1:500 for mumps, and 4–7 IU/mL for rubella
In addition, measles IgG antibodies in oral fluids were measured by capture EIA (Microimmune), in accordance with the manufacturer’s instructions. The cutoff OD value for seropositivity was set by the negative controls (mean × 1.25). Measles antibodies were also tested by the hemagglutination inhibition (HI) test, as described elsewhere [16]. HI titers ⩾1:5 were regarded as positive
Statistical methodsThe significance of differences between geometric mean antibody levels in different study groups was determined by an unpaired t test, and differences in antibody levels between sequential samples in the same group were tested by a paired t test, using the SPSS program (version 13.0)
Results
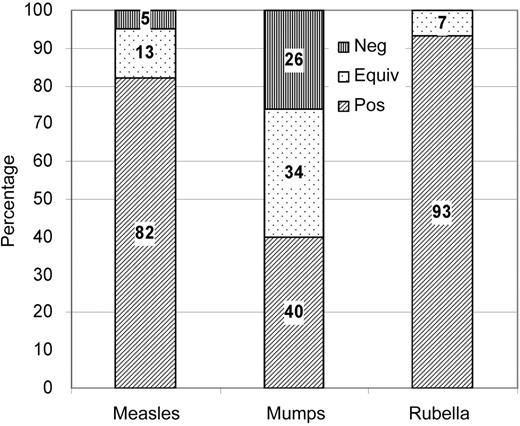
Antibodies against measlesThe seropositivity rate in younger vaccinees (n=85) for measles was 95% (95% confidence interval [CI], 91.4%–99.6%) at the 20-year follow-up time point in 2002 (figure 2), and 13% of vaccinees had an equivocal measles antibody level. The geometric mean level of antibodies to measles was 760 mIU/mL (range, 75–8400 mIU/mL) in younger vaccinees (n=85) and was 1113 mIU/mL (range, 220–5900 mIU/mL) in older vaccinees (n=6) (table 1). Among the originally seropositive vaccinees, the measles antibody levels were higher in the younger age group (n=6) than in the older age group (n=86). Statistical comparisons were not possible because there were too few samples in some subgroups. Ten (12%) of 85 originally seronegative vaccinees had measles antibody levels <200 mIU/mL, a putative protective level [19]
Proportion of seropositive (pos), equivocal (equiv), and seronegative (neg) results for antibodies against measles, mumps, and rubella in initially seronegative younger vaccinees (n=85 for measles, n=90 for mumps, and n=90 for rubella). Antibody levels were measured by EIA (Enzygnost; Dade Behring) 20 years after the first and 15 years after the second measles-mumps-rubella vaccination
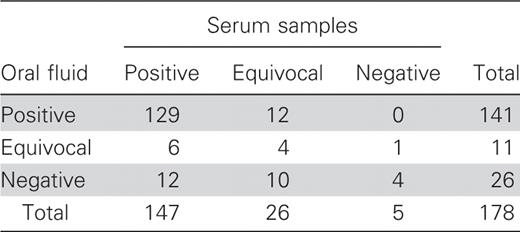
All of the 20-year follow-up samples were also tested by the measles HI test. On the basis of the test results (and considering HI titers <1:5 as negative), the measles seropositivity rate in younger vaccinees was 98%, somewhat higher than the rate based on EIA results; however, an additional 6% of the vaccinees had a very low antibody level, with an HI titer of 1:5. The positivity rate for measles antibodies measured from oral fluids was 85%, compared with 95% for serum samples. Of patients with negative oral fluid samples, 46% had positive and 39% had equivocal serum samples (table 2)
Concordance between measles antibodies measured in serum samples and oral fluids of vaccinees 15 years after the second measles-mumps-rubella vaccination
Antibodies against mumpsOf 90 initially seronegative younger vaccinees, 74% (95% CI, 65.0%–83.1%) had measurable antibodies against mumps 15 years after the second MMR dose (figure 2). The proportion of vaccinees with an equivocal mumps antibody titer was 34%. There was no significant difference between the geometric mean titers of mumps antibodies in younger (n=90) and older (n=73) vaccinees, which were 1:404 (range, 1:34 to 1:9200) and 1:422 (range, 1:57 to 1:2500), respectively. Antibody levels in the 2 initially seropositive age groups could not be compared because there were too few individuals (table 1)
Antibodies against rubellaAll of the 90 initially seronegative younger vaccinees were seropositive for rubella at the 20-year follow-up time point. An equivocal level of antibodies against rubella was found in 7% of vaccinees (figure 2). The geometric mean antibody level in seronegative younger vaccinees (n=90) was significantly lower than that in older vaccinees (n=74): 22 IU/mL (range, 4.6–130 IU/mL) versus 40 IU/mL (range, 19–260 IU/mL) (P<.001). Antibody levels could not be compared between the 2 initially seropositive age groups because there were too few individuals (table 1). Of the 90 initially seronegative vaccinees, 32 (36%) and 15 (17%) had rubella antibodies less than the putative protective levels of 15 and 10 IU/mL [20, 21], respectively
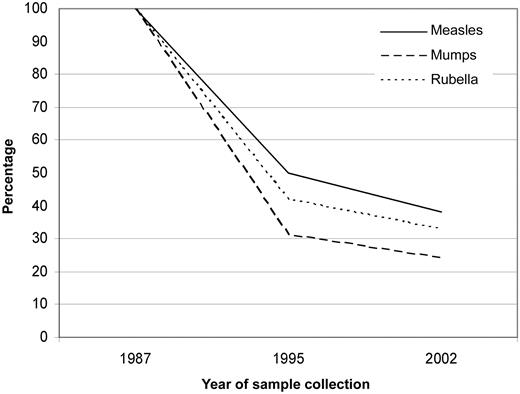
Kinetics of antibody declineAntibody decline was determined using samples collected from the 58 younger vaccinees initially seronegative for all measles, mumps, and rubella viruses 1 (1987), 8 (1995), and 15 (2002) years after the second MMR dose. The geometric mean level of antibodies to measles declined from 1917 to 957 to 729 mIU/mL during this follow-up. The geometric mean titer for mumps decreased from 1:2491 to 1:767 to 1:597 at these follow-up time points, and the rubella geometric mean level decreased from 67 to 28 to 22 IU/mL. During the first 8 years after the second dose (1987–1995), the decline in levels of antibodies against all 3 viruses was significant (P<.001); the decline was 50%, 69%, and 58% for measles, mumps, and rubella, respectively (figure 3). From then on, the antibody decline was substantially smaller but still significant: 23% for measles, from 957 to 729 mIU/mL (P<.001); 22% for mumps, from 1:767 to 1:597 (P<.001); and 21% for rubella, from 28 to 22 IU/mL (P<.05). The mean antibody decay rate during the 8 years after the second MMR vaccine was 7.1%, 9.9%, and 8.2% per year for measles, mumps, and rubella, respectively. Subsequently, the mean decay rate was slower: 3.5%, 3.2%, and 3.1% per year for measles, mumps, and rubella, respectively
Measles, mumps, and rubella IgG antibody levels were measured in samples collected from 58 vaccinees 1, 8, and 15 years after the second measles-mumps-rubella vaccination. The decline in geometric mean antibody levels is shown as the percentage of change from the level in 1987 (100%) to 1995 and to 2002, for measles (solid line) mumps (dashed line) and rubella (dotted line)
Antibody levels by sexThe antibody levels of originally seronegative female (n=27) and male (n=31) vaccinees did not differ significantly from each other soon after the second vaccine dose; the mean antibody levels in female versus male vaccinees were 2080 versus 2288 mIU/mL for measles, 1:2496 versus 1:2486 for mumps, and 65 versus 68 IU/mL for rubella. Subsequently, measles and mumps antibodies were higher in female than in male vaccinees. For measles antibodies, the geometric mean level for female versus male vaccinees were 1073 versus 866 mIU/mL (P=.25) 8 years after the second dose and 811 vs. 664 mIU/mL (P=.23) 15 years after the second dose. For mumps antibodies, the equivalent geometric mean levels were 1:942 versus 1:641 (P<.05) and 1:725 vs. 1:504 (P=.05), respectively. Rubella antibodies were at the same level in female and male vaccinees both 8 and 15 years after the second dose: 28 versus 28 IU/mL and 22 versus 23 IU/mL, respectively
Antibody levels in samples collected from 3 citiesAntibody levels were measured in 65, 59, and 59 serum samples from vaccinees in the 3 study cities, Joensuu, Pietarsaari, and Ivalo, respectively. The levels of antibody against all measles, mumps, and rubella viruses were lowest in the samples from the vaccinees originating from Ivalo, which is located in the northern part of Finland. The mean antibody levels in samples from Joensuu, Pietarsaari, and Ivalo were 1061, 658, and 533 mIU/mL, respectively, for measles; 1:452, 1:415, and 1:306 for mumps; and 29, 21, and 13 IU/mL for rubella. The difference between Joensuu and Ivalo was significant for rubella (29 vs. 13 IU/mL; P<.005) but not for measles (1061 vs. 533 mIU/mL; P=.088) or mumps (1:452 vs. 1:306; P=.16)
Discussion
In the present study, we have shown that, 15 years after the second dose of the MMR vaccine, the rate of seropositivity in initially seronegative vaccinees was 95%, 74%, and 100% for measles, mumps, and rubella, respectively. However, the geometric mean levels of antibodies against all 3 viruses declined significantly from the levels achieved after the second MMR dose, although with a slower rate of decline during the last 7 years
In our previous studies, measles and rubella antibodies were found in all and mumps antibodies in 95% of vaccinees soon after the second MMR dose [15–17]. Fifteen years after the second MMR vaccination, the rates of seropositivity to measles and rubella continue to be high, whereas the rate of seropositivity to mumps has decreased substantially. Measles and rubella seropositivity rates have remained sufficiently high, reaching the thresholds of 95% and 85%, respectively, for preventing measles and rubella virus circulation in the population [22]. The seropositivity threshold of 90% calculated as being necessary for preventing mumps virus circulation was not met 15 years after the second MMR dose. Such a high mumps seropositivity rate was measured only soon after the second MMR dose in 1987 [17], but levels were already below this threshold 8 years after the second dose
In several studies [23–25], as in our cohort, measles seropositivity has been shown to remain high for a long time, even as long as 33 years after vaccination [7]. Recently, LeBaron et al. [26] reported a measles seropositivity rate of 100% 10 years after the second MMR vaccination, measured by a sensitive neutralization test. However, in some of the studied countries, exposure to wild viruses may have boosted the vaccine-induced antibody levels [14]. Our cohort had theoretically been prone to natural measles, mumps, and rubella only during the 1980s, although the incidence of all 3 diseases rapidly decreased after the MMR campaign was launched in 1982 [3]. Since the beginning of the 1990s, no natural boosters of measles, mumps, and rubella have been available [1, 2], so the second half of the 20-year follow-up period has been free of indigenous measles, mumps, and rubella in Finland
In contrast with general opinion, our old reference method, the HI test (used since the beginning of our antibody follow-up), showed a slightly higher measles seropositivity rate than did the EIA, 98% vs. 95%. This difference could be attributed to a relatively high starting dilution used in the EIA test (1:231) compared with the HI test (1:5). Oral fluid samples provide a good means to determine antibody prevalence [27]. However, the lower total IgG content in oral fluid compared with serum leads to lower seropositivity rates—85% for measles in our study
A relatively large number of our vaccinees have become seronegative to mumps during the follow-up period. The proportion of low-level (equivocal) antibodies against mumps was higher than for measles or rubella (figure 2). However, the EIA used may have influenced the mumps seropositivity rate, because it is known to have a lower sensitivity than the neutralization test and tends to overestimate equivocal and negative results [28]. In addition, the mumps component of the trivalent MMR vaccine seems to be the weakest of the 3 antigens in inducing humoral immunity [10, 24, 29]. At any rate, our recent study shows that cellular immunity to mumps persists at least 21 years after the first MMR vaccination and is not dependent on antibody status [30]
Seropositivity to rubella has persisted at a very high level throughout the entire 20-year follow-up period, in spite of the significant waning of levels of antibodies against rubella. A similar long-term high rate of seropositivity to rubella has also been reported by O’Shea et al. [31] and Bottiger et al. [6], even after 1 vaccine dose. An interesting finding that we could not explain was that rubella antibody levels were significantly higher in the older than in the younger vaccinees in our cohort
The present study confirms the continuous waning of vaccine-induced immunity already seen in our previous studies as well as in other studies [8, 32]. Fifteen years after the second MMR vaccination, the mean antibody levels were roughly one-third of the levels measured right after the second dose (figure 3). The rate of antibody decline was rapid soon after the second dose (figure 3), and the decline happened simultaneously with decreasing circulation of wild virus in Finland. During the last 7 years of the follow-up (from 1995 to 2002), the antibody decline has been relatively slow, possibly predicting a long persistence of low-level antibodies. The majority (88%) of vaccinees still had a protective level (⩾200 mIU/mL) of measles antibodies. Although all vaccinees were seropositive for rubella antibodies, a protective antibody level was found in 64% or 83% of vaccinees, depending on the selected cutoff for protection (15 or 10 IU/mL). To date, an antibody level for protection against mumps has not been determined. Regardless of the declining antibody levels during the past 10 years—the period without indigenous measles, mumps, or rubella in Finland—a few imported cases of these diseases have not caused any outbreaks. This can be regarded as a sign of good herd immunity achieved by the high (>95%) vaccination coverage
We did not find any difference in antibody levels between sexes soon after the second MMR dose. Eight years later, however, antibody levels for measles and mumps, but not rubella, were lower in male than in female vaccinees, but the difference was significant only for mumps antibodies. Whether this finding has any implications for population immunity or epidemiology will be seen in the future
An epidemiologically interesting result is that the antibody levels were the lowest in vaccinees from Ivalo, in the north of Finland, particularly for antibodies against measles and rubella. In addition, all 4 younger vaccinees who were seronegative to measles came from Ivalo. Of the 3 cities studied, Ivalo has the lowest population density, which could partly explain this finding, as natural subclinical boosting (in the 1980s) has been more probable in Joensuu and Pietarsaari. The other possible reason for this difference could be a different genetic background for people in Ivalo compared with other Finns [33]
Outbreaks of measles and mumps continue to occur in many countries, even among vaccinated populations [34–38]. Although 2 vaccine doses have been shown to give better protection against measles than a single dose [39], in a recent mumps outbreak in the United States nearly half of the those affected had been vaccinated twice [40, 41]. Outbreaks of rubella among vaccinated populations have been very rare and limited in size [42], indicating good protection induced by the rubella strain RA27/3. The outbreaks usually develop as a consequence of a single imported case in a population with nonoptimal vaccination coverage. These reports are alarming even when vaccination coverage remains high in our country
At present, 40% of the Finnish population rely solely on vaccine-induced protection against measles, mumps, and rubella. Continuous follow-up of the humoral and cellular immunity in our unique cohort and of the antibody levels in the entire population is required to evaluate the possible need for changes in our current vaccination policy. We have shown that the measles, mumps, and rubella elimination target can be reached with sufficiently high coverage by the 2-dose MMR vaccination program. However, based on the results of this study, the waning of MMR vaccine–induced antibody levels may indicate difficulties in maintaining the achieved elimination in Finland as long as exposures from countries that have not yet eliminated these diseases continue
Acknowledgment
We thank Seija Salmi for performing the hemagglutination inhibition tests
References
Potential conflicts of interest: none reported
Financial support: National Public Health Institute, Helsinki, Finland